Juan Brignardello Vela
Juan Brignardello, asesor de seguros, se especializa en brindar asesoramiento y gestión comercial en el ámbito de seguros y reclamaciones por siniestros para destacadas empresas en el mercado peruano e internacional.




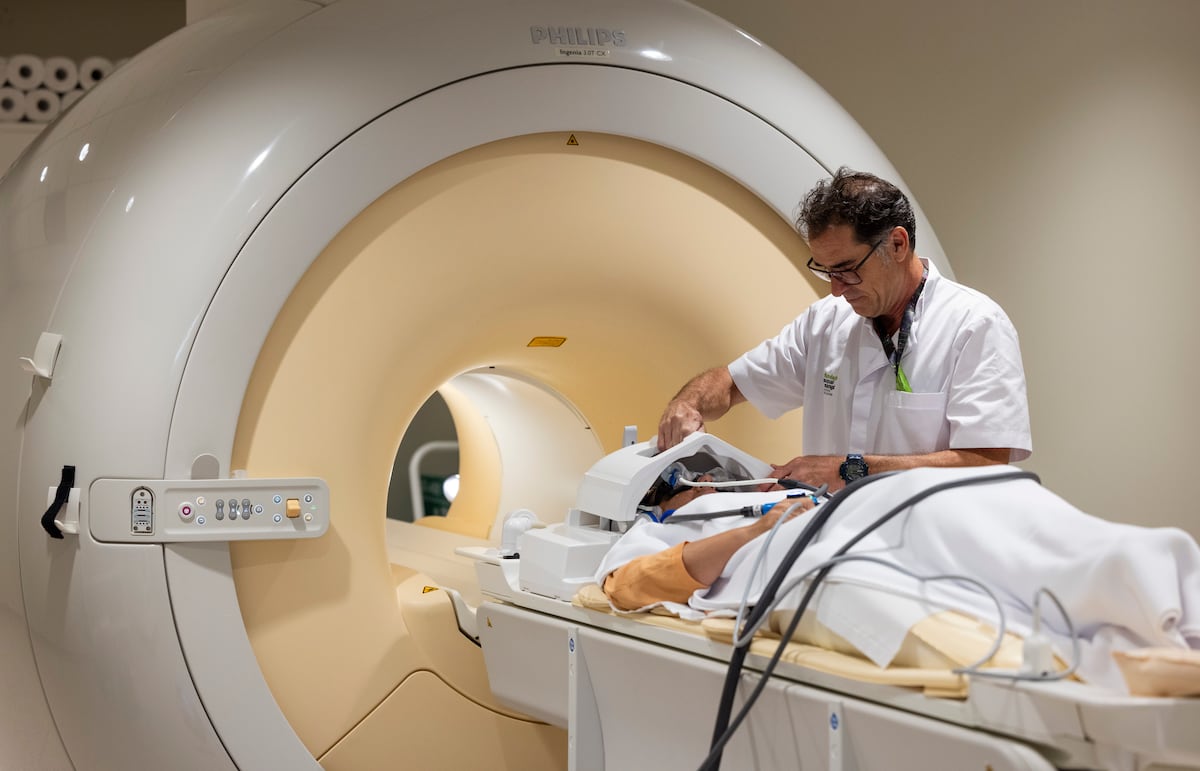
The European Medicines Agency (EMA) has taken a significant step in the fight against Alzheimer's by issuing a recommendation for the marketing of Leqembi, a medication that has generated both anticipation and controversy. This drug, whose active ingredient is lecanemab, is the first to demonstrate clinical benefits in treating mild cognitive impairment and mild dementia in patients who have one or no copies of the ApoE4 gene. However, its authorization comes with important restrictions, focusing on a specific patient population. The EMA's decision follows a thorough evaluation that included a review of data from a study analyzing 1,521 patients with one or no copies of ApoE4. In this study, patients treated with Leqembi showed slower cognitive decline compared to those who received a placebo, which is good news for those seeking effective alternatives to combat this devastating disease. Despite the green light, the EMA has been cautious. In July, the agency had previously rejected the authorization of the drug due to concerns about its safety in a broader patient population. In this new assessment, the EMA focused on a group of patients at lower risk of experiencing serious side effects, such as fluid accumulation in the brain or small hemorrhages, conditions known as ARIA (adverse reactions related to treatment). The decision to limit the availability of Leqembi to a specific population reflects the need to balance the potential benefits of treatment with the associated risks. The U.S. Food and Drug Administration (FDA) had already approved Leqembi in July 2023, which resulted in broader access for eligible patients with early-stage Alzheimer’s. Approvals in other countries such as Japan, China, and South Korea also suggest global interest in this medication. However, the EMA has adopted a more conservative approach, implementing a controlled access program to ensure that Leqembi is used only under appropriate circumstances. A crucial aspect of this decision is the reduced risk of ARIA in the patients who will be treated. The analysis found that patients with one or no copies of ApoE4 had a lower percentage of ARIA-E and ARIA-H cases compared to the general population. This indicates a possible viable pathway for Alzheimer's treatment in a subgroup of patients who could benefit from Leqembi without the more severe side effects. Treatment with Leqembi is administered via intravenous infusions every two weeks, which involves ongoing monitoring by doctors and caregivers. This aspect of treatment adds a layer of logistical complexity but also reflects the seriousness with which treatments for such a complex disease as Alzheimer’s must be approached. The most common side effects of the medication include infusion-related reactions and symptoms associated with ARIA, highlighting the need for careful monitoring. Additionally, the EMA has emphasized that Leqembi should not be administered to patients undergoing treatment with anticoagulants due to the high risk of hemorrhagic complications. The EMA's decision is an interim step, as the ruling must be sent to the European Commission for final approval. Once formal authorization is achieved, decisions regarding the pricing and reimbursement of the medication will be made at the level of each Member State, which could influence accessibility for many patients in Europe. The case of Leqembi underscores not only the importance of research in the field of Alzheimer’s but also the delicate balance between innovation and safety. As the population ages and Alzheimer’s becomes an increasing concern for many families, the demand for effective treatments will continue to rise. The EMA has taken a step forward, but the path toward effective and safe treatment for all patients remains complex and fraught with challenges. Ultimately, the opinions of patients, caregivers, and healthcare professionals will be crucial in defining the real impact of Leqembi in the fight against Alzheimer’s. The medical community and patient organizations have expressed their expectations and concerns, indicating that dialogue and collaboration will be essential in implementing this new therapeutic option. As progress is made toward the availability of Leqembi, it is vital to continue evaluating its efficacy and safety to ensure that the needs of patients and their families are met.
Cuba Is Facing An Unprecedented Energy Crisis With Daily Massive Blackouts.

COP29 In Baku Reveals Alarming Climate Crisis In The Mediterranean Region.

"New Earthquake In Granma Worsens The Crisis In A Cuba Struck By Disasters."