Juan Brignardello Vela
Juan Brignardello, asesor de seguros, se especializa en brindar asesoramiento y gestión comercial en el ámbito de seguros y reclamaciones por siniestros para destacadas empresas en el mercado peruano e internacional.




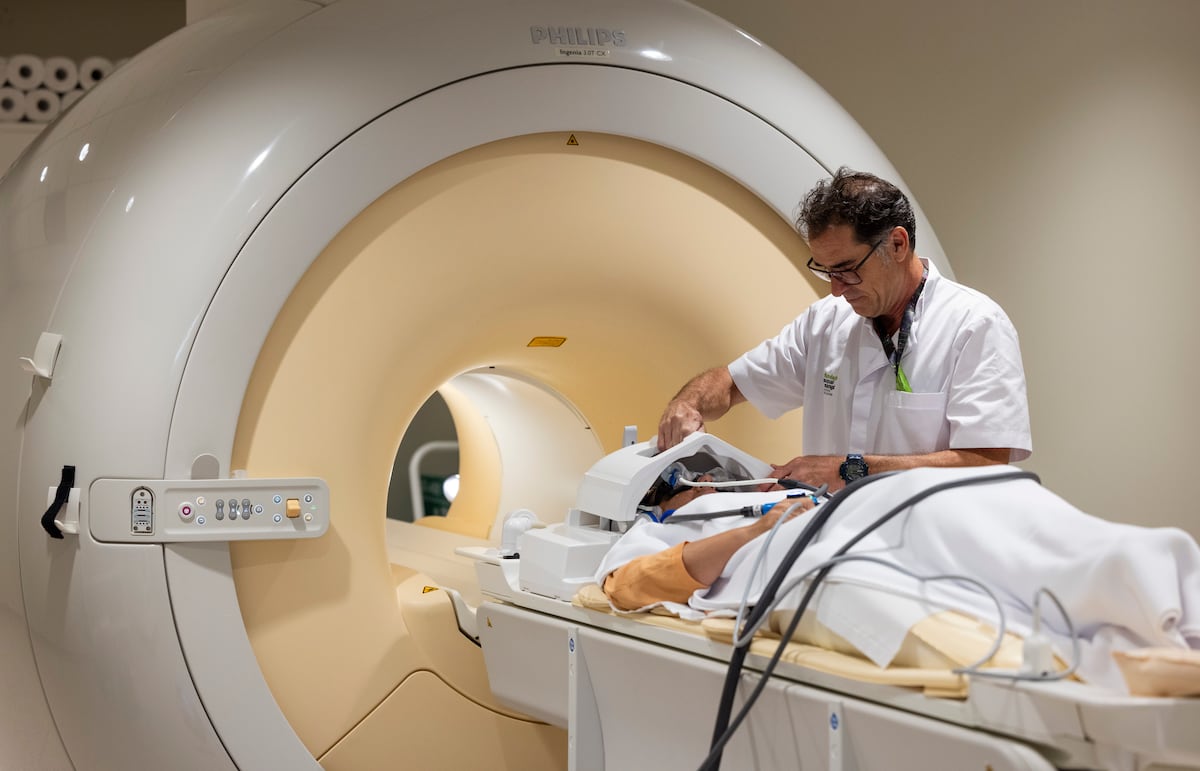
The recent decision by the Committee of Experts on Medicinal Products for Human Use of the European Medicines Agency (EMA) has opened a new chapter in the treatment of Alzheimer's in Europe. This Thursday, the committee recommended the approval of lecanemab, a drug that has generated both hope and controversy in the medical community and among patients. Considered the first treatment to have demonstrated some positive effect against this devastating disease in decades, lecanemab has been the subject of debate due to its side effects and high cost. The EMA had initially rejected lecanemab during the summer, but after an appeal from the manufacturing companies, the agency re-evaluated the data and concluded that the drug may have a place in the treatment of Alzheimer's, albeit with restrictions. Its use is recommended only for patients with no more than one copy of the APOE4 gene, which increases the risk of inflammation and bleeding associated with the treatment. This decision excludes approximately 15% of patients who could benefit from the drug. Pending formal approval from the EMA, which is expected to happen, each country in the European Union will need to negotiate the price of lecanemab before deciding whether to include it in their public health system. In the United States, where the drug has already been approved, its cost is approximately $26,500 per year, which translates to about €24,000. The decision on public funding for this drug will be crucial, given concerns about its cost and the limited effectiveness it has demonstrated. Lecanemab, marketed as Leqembi, has shown the ability to delay the progression of Alzheimer's by 27% in clinical studies involving 1,700 patients across several countries, including Spain. However, the concrete impact of this delay on the quality of life of patients and their caregivers remains an open question, especially considering that the drug has caused significant adverse effects, such as brain inflammation in 12% of patients, with fatal consequences in two cases. The companies that developed the drug, Eisai and Biogen, have presented additional data suggesting a continuous improvement in the cognition of patients who remain on treatment. However, this data has yet to undergo independent review, leaving its validity and applicability to a broader population in question. With the recommended approval of lecanemab, Europe joins a list of countries that have already green-lighted its use, including the United States, Japan, and several Asian countries. However, the United Kingdom has decided not to fund the drug through its public health system, arguing that the benefits are too modest compared to the high cost it would entail. Access to lecanemab will be controlled through a specific program that ensures its use only in the indicated population. Patients will need to undergo periodic MRIs to detect any inflammation before and during treatment. The EMA has also mandated that pharmaceutical companies provide adequate training for healthcare professionals and establish a patient registry to monitor the drug's side effects. Alzheimer's, which affects nearly 50 million people worldwide, presents a growing challenge due to the aging population. Projections suggest that the number of affected individuals could increase dramatically in the coming decades. However, while expectations are high, it is estimated that only about 2.5% of the 800,000 Alzheimer's patients in Spain could benefit from lecanemab under its restricted use. This drug interacts with amyloid protein plaques in the brain, which are believed to be responsible for the disease. To be effective, it must be administered to individuals in very early stages of the disease, when they still have some independence. This presents significant challenges for healthcare systems, which will need to manage complex diagnostic testing and frequent intravenous treatments, potentially increasing the total treatment cost per patient significantly. Experts in the field, such as Bart De Strooper, co-founder of the UK Dementia Research Institute, have expressed satisfaction with the change in decision in Europe, considering it a significant advancement. Other neurologists, such as Juan Fortea from the Sant Pau Hospital in Barcelona, share this view, stating that the approval of lecanemab could catalyze the development of new diagnostic and therapeutic capabilities, benefiting not only those who receive the drug but all Alzheimer's patients in the future. Meanwhile, donanemab, another similar antibody that has shown a 35% reduction in the progression of Alzheimer's, is still under evaluation in Europe, leaving the door open for new hopes in the fight against this devastating disease. The story of lecanemab may just be the beginning of a paradigm shift in Alzheimer's treatment, provided that a cautious approach focused on patient safety is maintained.